Change Management considered harmful?
Posted by Dan Matheson on Tue, Jul 10, 2020 @ 10:36 AM
These comments are inspired by two things: 1) observations during PLM consulting work in life science companies and 2) remembering an article by Edeger W. Dijkstra, A Case Against the GO TO Statement, in Communications of the ACM 11 (1968), 3: 147–148. As an R&D product development engineer, I have found the following a great help to innovation: change the vocabulary to change the perspective on the problem, the new perspective enables new insights for innovation.
Let’s change the vocabulary. Most PLM systems have a Change Management module. Rather than thinking of Change Management, let’s use the term Improvement Management. For released products we do not make changes, we make improvements. Assuming a company wants to create “Safe, Effective, Profitable and Great” products, then improvements should be measureable in one or more of those four categories.
The measurements have a qualitative benefit and a quantitative benefit. The qualitative aspect is one of the four categories of Safe, Efficacy, Quality or Greatness. A proposed improvement should be able to describe in scientific or engineering terms the way in which a product will be better. Will the product be better because irritating chemicals are less, will it be more effective for the intended use because the stability of the product is increased, or will there be less waste in the manufacturing process.
The quantitative benefit is a definitive metric of the improvement in dollars and the corresponding cost to implement. These two numbers will allow the business to determine that the improvement will help the profitability of the company. The sources for improvements are the usual suspects, customer suggestions, competitor’s products, CAPAs, NCRs, new materials, new process technologies, etc. Just because the new vocabulary is “improvement” that does not mean that good processes like CMII cannot be employed to maintain quality.
What does change look like to the pre-released product? During the R&D phase the product definition is being established. A more productive vocabulary is to look at the R&D work as evolving or refining the product definition. This includes the manufacturing process. The evolution view means that simpler approval or review processes can be used to provide stability points during the R&D phase. The stability points can be used in conjunction with business gate reviews or to meet business requirements before clinical trials. The new perspective for the R&D phase is that the definition is fluid, but hopefully moving towards a stable release status.
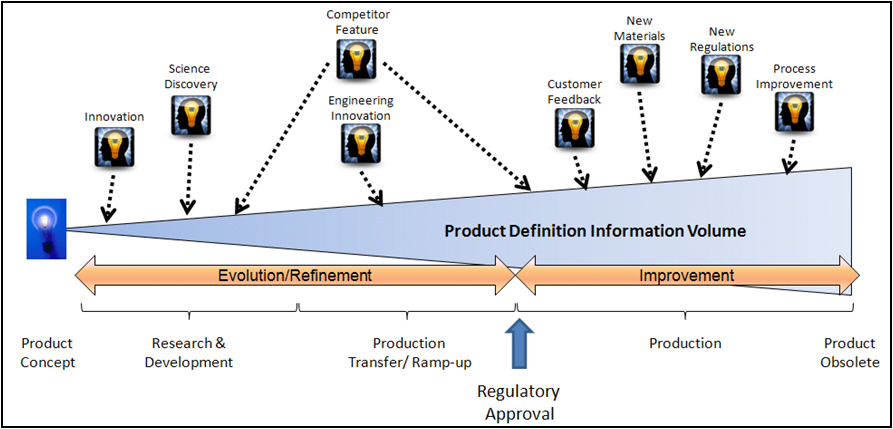
The release point for a product will be considered to be the regulatory approval to sell. Prior to release the product definition evolves and after release improvements are made. This new vocabulary helps support the old engineering saying of “If it ain’t broke, don’t fix it”.
The vocabulary of Improvement and Benefit to Safe, Effective, Profitable and Great products will help move the organizational mindset away from change. In many cases it has been observed that change comes from infatuation with new things or a localized view of cost savings rather than company profitability.
The word “change” is considered harmful.
Dan Matheson
Senior Architect, Integware