Key characteristics of an ideal QMS
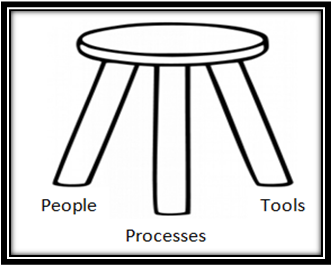 When building your Quality Management System (QMS) for your Medical Device Company, use this simple guide to develop the foundational three-legged-stool. Realize that building an ideal QMS takes more than a good quality manual. We must consider people, processes, and tools as critical components of a system that comes together in harmony.
When building your Quality Management System (QMS) for your Medical Device Company, use this simple guide to develop the foundational three-legged-stool. Realize that building an ideal QMS takes more than a good quality manual. We must consider people, processes, and tools as critical components of a system that comes together in harmony.
Our goal of building the ideal QMS is to ensure products are produced consistently to a level that satisfies the requirements of the product design and product development and usage process. Typically from both a safety and efficacy perspective (will it work) and does it work as we expected (post-market surveillance).
The following guide is very simple and covers the basic concepts of that ideal QMS.
This content is targeted somewhat to Medical Device Companies, however, most of it is good common sense that would work well in any organization taking quality seriously or adhering to current good manufacturing processes cGMPs.
