Justifying PLM: A New Perspective on the Investment for Life Sciences Companies
Posted by Richard Robbins on Fri, Apr 27, 2020 @ 12:56 PM
I have been calling directly into the Life Sciences (LS) space for about a year and a half. I will not call myself an expert, but given that I have been in the business world for over 30 years, I feel I have crossed over into the category of a “gray hair” and therefore I can have some relevant observations. My below observations are in some ways provided as an outsider looking into the LS industry.
PLM platform providers and services firms calling into the LS industry seem to focus heavily on how PLM can address FDA and other regulatory compliance requirements. Indeed there is a lot of value to represent, but I think there is a different – and potentially more compelling – value proposition to present to our prospective clients. Let me explain.
Many LS companies have initially addressed their QMS requirements through a “fire fighting” mode. They start primarily manual and paper based in their approach. A 483 or Warning Letter drops into their lap, and they react swiftly by addressing the specific observation (e.g. CAPA, Complaints, Document Control, etc.) with either a home grown approach or a non-PLM point solution offering in the market place. This addresses the immediate need, but it starts the legacy of “point solution proliferation”. Many times, we in the PLM market place enter our clients at this moment in time to discuss and educate our clients on the strategic power of end to end traceability and “single version of the truth” advantages that PLM provides an LS customer. Here is where our efforts are more often than not rebuffed because the prospective client had invested in a home grown or non-integrated point solution approach; changing direction to PLM would require very painful political realignment and admission that past decisions were not thought out strategically.
The education process on the financial advantages of an integrated PLM approach drags out for months/years, all the while the LS customer is losing a HUGE opportunity cost through the process (note: Integware just completed an engagement with a major med device manufacturer which concluded that for every month they delay implementing an integrated PLM approach vs. their current manual and point solution legacy approach, it costs their enterprise up to $500,000/month in benefits).
What if a PLM platform direction and investment were calculated on the impact it has on a company's ability to innovate, its ability to accelerate the new product development process and enhance and refine the existing products sold? As part of our recent strategy engagements at Integware, we are finding that the results from this perspective are off the charts impressive (> 50% average annual ROI).
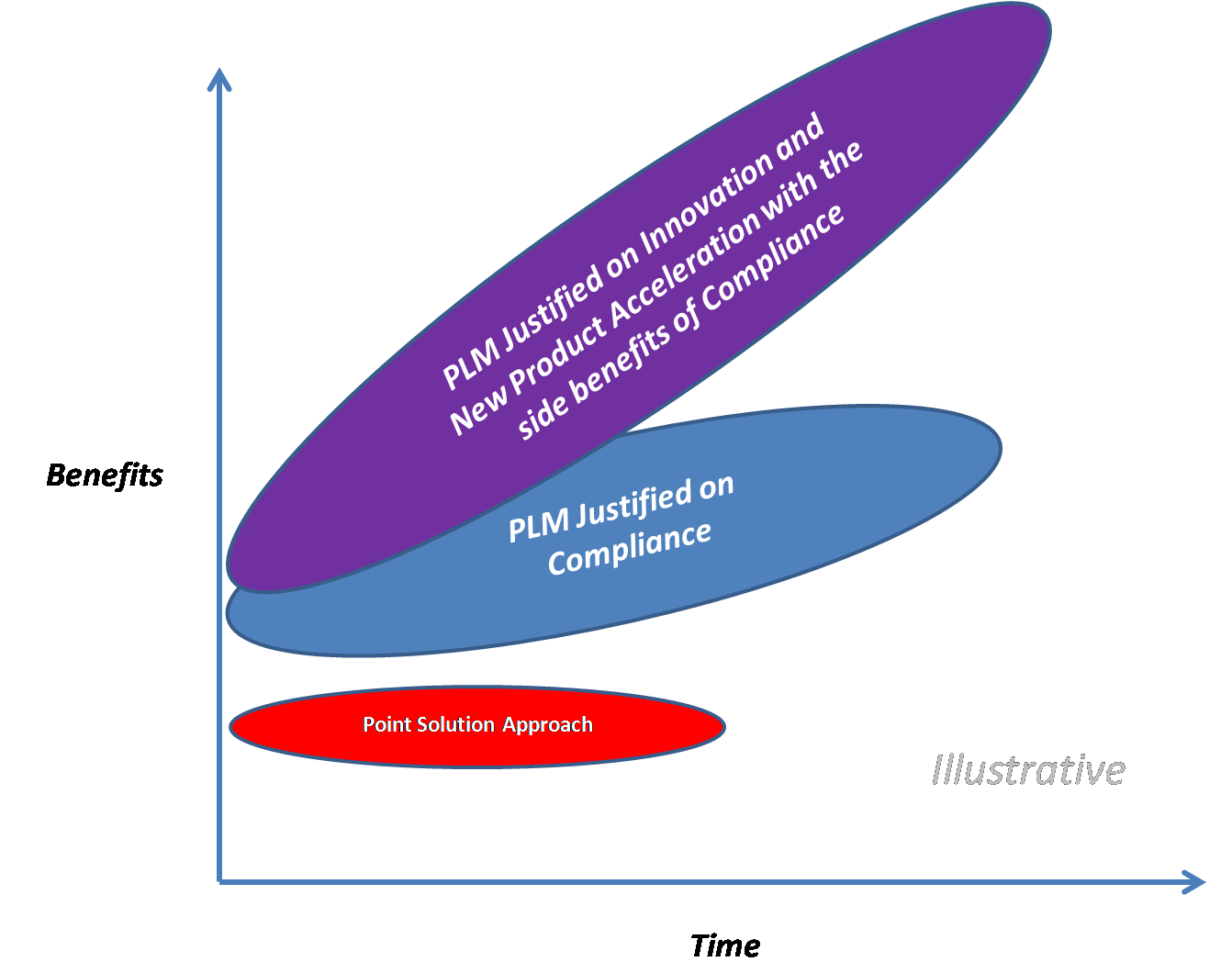
By implementing a truly integrated PLM approach with end to end traceability of data, from product ideation through post launch and end of life obsolescence, our clients are able to solidly (in financial terms for their C level executives and BOD’s) identify definable and material gains in:
- revenue & market share improvement
- cost reductions
- patient liability and regulatory risk reduction
- customer satisfaction
- expansion of business into adjacent markets
An integrated PLM approach lowers costs of regulatory compliance (i.e. faster FDA audit resolution, etc.) and increases quality of their products/services significantly, but the added benefit of innovation and market expansion seems to be the real prize. An integrated approach to the Total Product Lifecycle (also called TPLC by the FDA) is where the big benefits are to justify the investment, time and organizational change management required to implement enterprise wide PLM.
What are your thoughts on this perspective?